Fertility and The Three Sisters

At the time of writing, late July 2023, the local grain harvest is just starting. The combines will be out in the Winter Barley. But there is a long way to go and typically the weather has changed to a pattern of Atlantic lows giving us sunshine and showers from the west. In spite of a dry June, some say the hottest on record, predictions for wheat, barley, oats and oil-seed rape are good. Prices have dropped from last year and the high cost of electricity means that farmers will be anxious to use the combine in dry spells and avoid the cost of drying their grain before storing.
Few consumers are aware that farmers have to budget 18 months in advance. The fertiliser and other inputs for this year’s crop will have been purchased early last autumn and the crop may not be sold till next spring. Covid, Brexit and the Ukraine war have made it very difficult to plan ahead. The change from the EU basic payment scheme to ELMS has added further uncertainty. The cost of fertiliser, energy, animal feed and other inputs has yo-yoed and so have prices for arable crops . No wonder many farmers are suffering mental health problems!
Seeing all these large machines, combines and tractors rushing about may make you question the carbon impact of farming. Yes, farming has a huge carbon impact, but burning diesel is not the key issue. It is likely that hydrogen power will be introduced to phase out diesel on large machinery and small electric tractors and drones are already whizzing about on lighter tasks. Many farmers are also well-placed to create their own energy from bio-fuels, solar and other sources. No, the difficult issues are nitrous oxide and methane. I have done a little research into N2O (you can follow this at Ref 1 in the References at the end of the post) and I should like to write about methane another time. In addition, farmers are being accused of polluting water courses with nitrates and phosphates, so in this article, I have tried to explain why farmers apply fertiliser to their crops.
Nitrous Oxide (N2O)
Nitrous oxide is nearly 300 times more active as a greenhouse gas than carbon dioxide but occurs in very small quantities (approximately 0.32 ppm as opposed to 419 ppm for CO2). In some countries, agriculture releases 70 - 80% of total nitrous oxide emissions. However it is mainly a natural process of the incomplete breakdown of inorganic nitrogen compounds (ammonium, nitrite and nitrate) during denitrification and the return of Nitrogen to the atmosphere. Careful ‘little and often’ application of nitrogen fertiliser, including manure, and avoidance of water-logged conditions can help, but as a natural process, it is difficult to eliminate N2O completely.
Nitrogen Fertilisers
Whenever we remove a crop at harvest, we take with it major elements such as nitrogen, phophorus, potassium, calcium, magnesium, and sulphur, plus minor trace elements such as iron, boron, managanese etc. These have to be replaced if the soil is to remain fertile. I get quite angry when I hear journalists and others spitting out the phrase ‘artificial fertilisers’ with venom: this led me to re-visit the history of modern fertiliser, especially nitrogen, and why we need it.
Nitrogen makes up 78% of the atmosphere and is an essential component of amino-acids and proteins in living organisms. To get from one to the other, nitrogen gas has to be converted to nitrate (NO3-) or ammonium (NH4+), forms that plants can absorb – see the simple diagram of the Nitrogen Cycle below. Nitrogen is also made available by the breakdown of plants and animals and manure or ‘night-soil’. Other elements come mainly from rock particles carried in rivers (alluvial) or in air (loess).
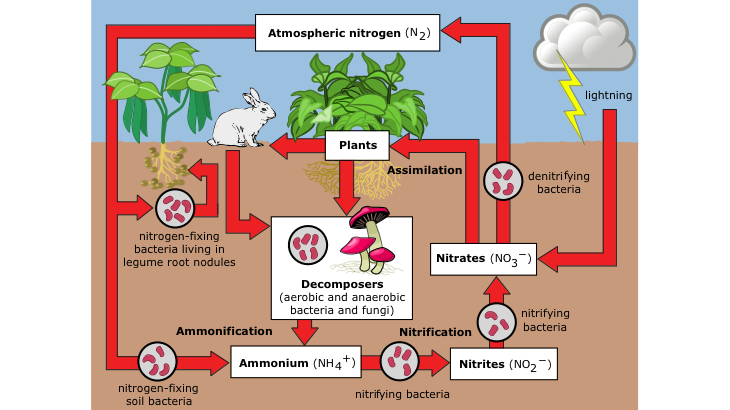
Since the Black Death, the world population has increased from about 400 million to 7.9 billion. Food production has kept pace although equable food distribution has not.
In the Middle Ages, land was fallowed with a ley (grass) that could be grazed. However, at the beginning of the 16th Century a four-crop rotation was developed in Waasland (modern Belgium) and adopted in England in the early 18th Century by ‘Turnip’ Townsend and colleagues in Norfolk. The rotation was wheat, turnips, barley and clover. There was no fallow; the turnips or clover could be grazed and the clover and animal manure contributed nitrogen to the soil. Until North America started exports, this system fed the early increase in UK population during the Industrial Revolution.
In the early 19th Century, small islands were discovered off the coast of Peru, home to millions of sea birds. The guano (bird poo) was up to 200 feet deep! It was lauded as the world’s finest fertiliser and ships from US and Europe were queuing up. But the supply was not sustainable and by 1870 was running out. Mining of Potassium Nitrate and Sodium Nitrate (both known as “Nitre” or "Saltpeter") in Chile could not keep pace either. Research in Germany led to the invention of the Haber-Bosch process for converting atmospheric Nitrogen to Ammonia - just in time for the First World War. As well as making good explosives, Ammonium Nitrate (NH4NO3) at 34%N is an excellent fertiliser.
Urea (CO(NH2)2) is the cheapest source of Nitrogen (46%) and more common in many countries than Ammonium Nitrate as it cannot be used for home-made bombs. Urea breaks down in the soil to give ammonium ions (NH4-). The ammonium is taken up by the plant through its roots or it is oxidized by bacteria to give nitrate (NO3-), which is also a nitrogen-rich plant nutrient. However, if urea is applied to the soil surface, a fraction may be lost to the atmosphere as ammonia gas.
Ammonium sulphate ((NH4)2SO4 ) contains 21% Nitrogen making it more expensive to transport, but it is not explosive, can contribute Sulphur where needed and is slightly acidifying – useful on chalk soils.
Organic growers are not allowed to use any of these three synthetic fertilisers. They can import guano, or naturally occurring potassium nitrate (13% N, 44% K), or sodium nitrate (16% N, 26% Na). But generally they are advised to use nitrogen fixing plants (legumes) or animal manures.
Worldwide the most popular legume crop is soya, but soya beans are not the best at fixing atmospheric nitrogen and most growers add some synthetic nitrogen fertiliser to maximise yields.
Gardeners may like to experiment with the ancient practice of Three Sisters Planting (3)
The sisters are normally maize (or sweetcorn or sunflower), climbing beans and pumpkins (or other squash). Once the maize is established, the beans are sown at the base and use the maize stems for support. The pumpkins have huge leaves which cover the ground to suppress weeds. Maize and pumpkins benefit from the nitrogen supplied by the beans. The problem for commercial farmers is that the three crops have to be harvested manually: we are a long way from robots that can sort out maize, beans and pumpkins in the field.
Organic vs Inorganic Nitrogen
Given that plants take up most of their nitrogen requirement from the soil as inorganic nitrate (NO3-), what is the problem with synthetic N fertilisers?
Problem 1 Carbon footprint
Firstly, the Haber-Bosch process relies on fossil fuels (natural gas) to produce hydrogen and the vast amount of energy for a high temperature/pressure reaction. The hydrogen is combined with atmospheric nitrogen to produce ammonia. So to manufacture ammonia in a climate-friendly way, manufacturers have to use renewable energy supplies and break down water by electrolysis for the hydrogen. My chemistry is not good enough to understand all the ins and outs of this and other possible routes, but there is a summary diagram below. (If you are interested try reading the Ref 4 article).
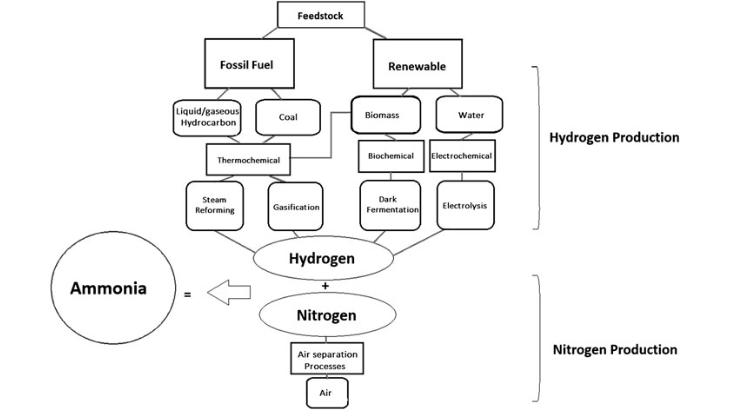
It is worth pointing out that ammonia is not only important in agriculture, but has many other uses including the longer term storage of energy from renewables.
Problem 2 - Fertiliser application
Second, there is the problem of accurate application of fertilisers. Nitrates are highly soluble and are one of the chief pollutants of our rivers. Applying too much nitrogen at the wrong time of year leads to surface run-off and leaching through the top-soil into aquifers. Farmers get advice on fertiliser application from RB209. I used this ministry booklet in the 1970s; it is in its 50th year and it is brought up to date regularly. You can read it on-line (5). It relies on soil analysis, rainfall data and crop uptake. It gives the nutrient content of all types of fertilisers and manures.
One of the recent changes is in nitrogen calculations. Because of its solubility, simple soil analysis which works well for P (Phosphorus), K (Potassium) and Mg (Magnesium), does not work for N (Nitrogen). Fields vary widely in the amount of nitrogen available to a crop before any fertiliser or manure is applied. This variation must be taken into account to avoid inadequate or excessive applications of nitrogen. RB209 uses the soil nitrogen supply (SNS) system which assigns an Index of 0 to 6 to indicate the likely extent of this background nitrogen supply. The Index is used to select the amount of nitrogen, as manufactured fertiliser, manure or a combination of both, that typically would need to be applied to ensure optimum yield. The SNS Index for each field can be estimated either by the Field Assessment Method using records of soil type, previous cropping and winter rainfall, or by the Measurement Method. This uses measurements of soil mineral nitrogen plus estimates of nitrogen already in the crop (at the time of soil sampling) and of available nitrogen from the mineralisation of soil organic matter and crop debris during the period of active crop growth. These nitrogen calculations can be used by conventional farmers using artificial fertiliser or by organic farmers applying farmyard manure.
High tech precision farmers may use GPS to vary the application of nitrogen as the tractor works the field. Photographic information from drones can assess the health of the crop in advance. This information is used to change the rate mechanism of the spreader.
Wheat farmers will balance the cost of fertiliser against the likely yield gain, but they also have to take into account the quality of the wheat to get the highest prices. Some farmers are finding that they make more money using less fertiliser even if there is a yield penalty.
As a fruit grower, we used regular leaf analysis for N through the growing season. Too little N and the fruit buds are weak and there is not enough new wood growth. Too much N and the apples are soft, colourless and do not store well. As apples contain little nitrogen, they require little nitrogen. Instead of applying it to the soil, we often applied it to the leaves with a dilute spray of urea before blossom. We also applied urea sprays after harvest to ensure that the leaves rotted quickly without allowing diseases like scab to continue into the next season.
Problem 3 – Nitrogen leaching
The third aspect of nitrogen management is control of soil levels throughout the year, especially in the autumn. During the summer, higher soil temperatures increase the rate of microbial activity, nitrifying bacteria and nitrogen fixing bacteria are busy. By the end of the summer, soil nitrates are high. Fifty years ago, wheat growers applied nitrogen to the soil when drilling the crop in the autumn. Research has shown this to be wasteful and unnecessary. In fact the reverse is often needed and this is where ‘cover’ crops are used. The objective is to avoid leaving the soil bare and to avoid autumn rains washing soluble nitrate into the drains and rivers. Some cover crops are sown into the standing wheat before combining; some are drilled into the stubble after combining. The cover crop mops up surplus nitrogen and is grazed, mown, weed-killed or ploughed-in according to soil type and the timing for drilling the following crop. With high fertiliser prices, farmers value this free source of nitrogen.
By February if there is a warm spell and after high winter rainfall, wheat crops can start to look quite yellow indicating a shortage of nitrogen. From then on, nitrogen is applied ‘little and often’ as recommended by FACTS trained crop advisers.
Problem 4 – Manure in rivers
The fourth cause of nitrogen pollution is from the spreading of slurry and farmyard manure (FYM). Slurry is wet animal manure without straw. Because the law prevents it being spread at certain times of the year, storage is a problem. Slurry lagoons are expensive and often leak. Inspections by the Environment Agency and Red Tractor are not frequent or strict enough to prevent this happening, but small livestock farmers often do not have the money to rectify matters. Recently incidents from Devon reached the news. There is currently also a massive problem with pollution of one of our iconic rivers, the River Wye, caused by a ‘cluster’ of chicken farmers on the Welsh borders with insufficient land on which to spread their chicken manure. Of all organic manures, chicken manure is highest in nitrogen. These manures might be welcome on the arable lands of East Anglia, but the problem is getting it there. FYM is bulky and costly to transport. The alternative might be to use it in bio-digesters to produce energy.
Conclusion
I have gone into some detail about crop nutrition, especially nitrogen. You may not have read or understood it all, but the important point is that it is highly technical and with the emphasis on good soil management, climate action, and producing at least 60% of our own food, there is a lot to get right. Scientists are using new methods to understand soils better and farmers are realising that soil is a key asset and that they have to contribute to net zero as promised. We have to stop polluting and destroying our rivers.
Organic matter and soil carbon are important, but searching on-line, I could not find any scientific evidence that fertilisers are destroying our soils if managed correctly. I suspect that bunging on some manure and hoping for the best may be good enough for the garden but it is not going to feed the nation! Let me know how you get on with the three sisters in your allotment or at home – I shall definitely be trying it next year.
REFERENCES
1 You can follow all this on https://ahdb.org.uk/markets-and-prices
2 Wikipedia: The Nitrogen Cycle - Figure derived from: Nitrogen Cycle.svg:, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=80960223
3 https://www.rhs.org.uk/advice/grow-your-own/features/three-sisters
4 Frontiers in Energy Research Vol 9 2021 - Sustainable Ammonia Production Processes
https://www.frontiersin.org/articles/10.3389/fenrg.2021.580808
5 Agriculture and Horticulture Development Board - Nutrient Management Guide (RB209)
https://ahdb.org.uk/nutrient-management-guide-rb209
6 BASIS Registration Ltd - Scheme FACTS
https://basis-reg.co.uk/scheme-facts

